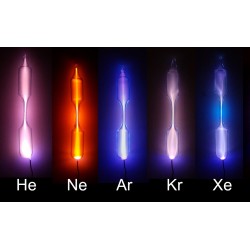
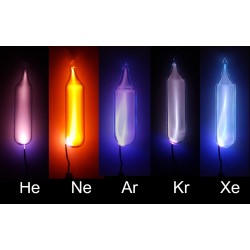
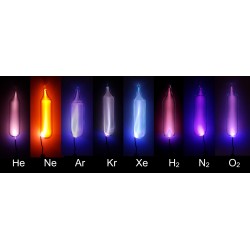
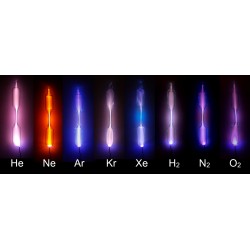
Helium
Helium, represented by the chemical symbol He, is a noble gas and the second-lightest element in the periodic table. Its discovery is attributed to the French astronomer Jules Janssen and the English astronomer Norman Lockyer in 1868, independently. However, it was the Scottish chemist Sir William Ramsay who successfully isolated helium on Earth in 1895.
Despite its cosmic abundance, helium is scarce on Earth, constituting only about 0.0005% of the atmosphere. It is primarily obtained as a byproduct of natural gas extraction, and the United States holds significant helium reserves. Helium's low boiling point and non-reactive nature make it invaluable in various applications. Its use in cooling applications, such as cooling superconducting magnets in magnetic resonance imaging (MRI) machines, is critical. Additionally, helium is employed in research and scientific experiments due to its stability and unique properties.
Looking to the future, helium's role in cooling technologies, especially in the field of quantum computing and high-tech applications, is promising. As advancements in technology continue, helium's importance is expected to grow, emphasizing the need for responsible helium conservation and exploration of alternative sources to meet the increasing demand.